
His theory of electron distribution inside an atom was later proved to be incorrect by Ernest Rutherford. Realized that if there ware negative parts to atoms, then there must be positive parts as well to balance it, because most matter is neutral. Won a noble prize for his great work on the electron. Compounds are formed by joining atoms in specific whole number ratios. Atoms are indestructible and retain their identity in chemical reactions. The atoms of one kind of element are different from the atoms of all other elements - in particular the atoms of one element have a different mass than those of other elements. All atoms of the same element are identical – in particular they have the same mass. Dalton’s Theory All elements are composed of atoms. Was one of the earliest scientists to contribute to the modern atomic theory. Thompson, Ernest Rutherford, Niels Bohr, and Ernest Schrodinger all contributed significantly to the modern atomic theory by finding the actual evidence.

Theories are imaginative ways to explain why something happens.ĥ Democritus Around 400 BC, a Greek philosopher named Democritus suggested the first atomic theory He explained that all things are "composed of minute, invisible, indestructible particles of pure matter which move about eternally in infinite empty Even though there was no technology to help research Democritus‘s theory, he was very precise John Dalton, J.J. They just describe and summarize what happens. They can be broken down into elements again by chemical means. Compounds are pure substances that are made up of two or more elements chemically combined together. Each element has its own distinct properties and cannot be broken down into simpler substances by means of a chemical change.
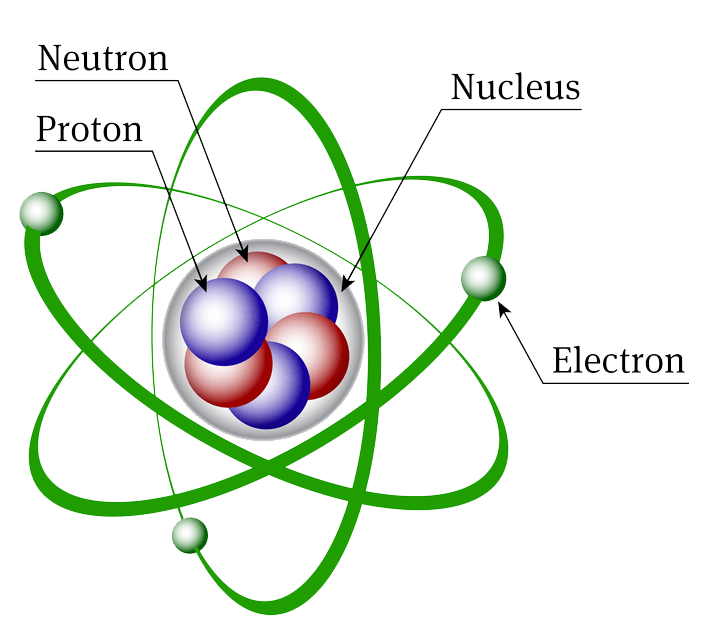
Compounds An element is a pure substance made up of one type of particle, or atom. This attraction holds the electrons around the nucleus.ģ Elements vs. Protons and electrons have opposite charges, therefore, they attract.
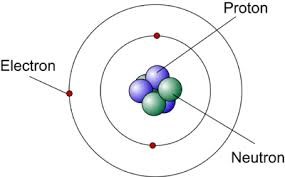
1 Modern Atomic Theory Grade 9 Science ChemistryĢ Modern Atomic Theory Atoms are made of 3 particles protons, neutrons and electrons Atoms have a nucleus that is made of protons and neutrons with electrons orbiting around it.


 0 kommentar(er)
0 kommentar(er)
